 Microneedle-based drug delivery has the potential to be a transformative technology for the delivery of biologics and vaccines. It may provide enhanced therapeutic profiles for therapeutics and vaccines. It allows for the administration of lower levels of drugs to achieve the same therapeutic endpoints. Additionally, microneedles provide an alternative to traditional hypodermic needles. This industry provides a means to overcome one of the biggest barriers to patient compliance and safety for the treatment of chronic diseases and routine vaccination. The variation in the microneedle types could also prove useful in controlling the kinetics of vaccine release. The importance of overcoming stratum corneum barrier is central to the efficient Microneedle-mediate transdermal and intradermal delivery. 1,2
Microneedle-based drug delivery has the potential to be a transformative technology for the delivery of biologics and vaccines. It may provide enhanced therapeutic profiles for therapeutics and vaccines. It allows for the administration of lower levels of drugs to achieve the same therapeutic endpoints. Additionally, microneedles provide an alternative to traditional hypodermic needles. This industry provides a means to overcome one of the biggest barriers to patient compliance and safety for the treatment of chronic diseases and routine vaccination. The variation in the microneedle types could also prove useful in controlling the kinetics of vaccine release. The importance of overcoming stratum corneum barrier is central to the efficient Microneedle-mediate transdermal and intradermal delivery. 1,2
 Several new transdermal products mediated by microneedle methods have already been marketed and a few others reach their late-stage development. According to “The Microneedles for Transdermal and Intradermal Drug Delivery, 2014-2030” report, more than 70% of the products in development are patches incorporating solid or dissolvable needles, the rest are hollow microneedle arrays that employ the use of a syringe. However, microneedle technologies have not reached their full maturity, yet due to a lack of cost-effective manufacturing technologies. With several new microneedle-based therapeutic product launches by the end of this decade, the report concludes that the overall market for microneedle-based delivery devices will reach annual sales of 485 million units by 2030. https://www.medicaldesignandoutsourcing.com/microneedle-patches-enable-superior-drug-delivery
Several new transdermal products mediated by microneedle methods have already been marketed and a few others reach their late-stage development. According to “The Microneedles for Transdermal and Intradermal Drug Delivery, 2014-2030” report, more than 70% of the products in development are patches incorporating solid or dissolvable needles, the rest are hollow microneedle arrays that employ the use of a syringe. However, microneedle technologies have not reached their full maturity, yet due to a lack of cost-effective manufacturing technologies. With several new microneedle-based therapeutic product launches by the end of this decade, the report concludes that the overall market for microneedle-based delivery devices will reach annual sales of 485 million units by 2030. https://www.medicaldesignandoutsourcing.com/microneedle-patches-enable-superior-drug-delivery
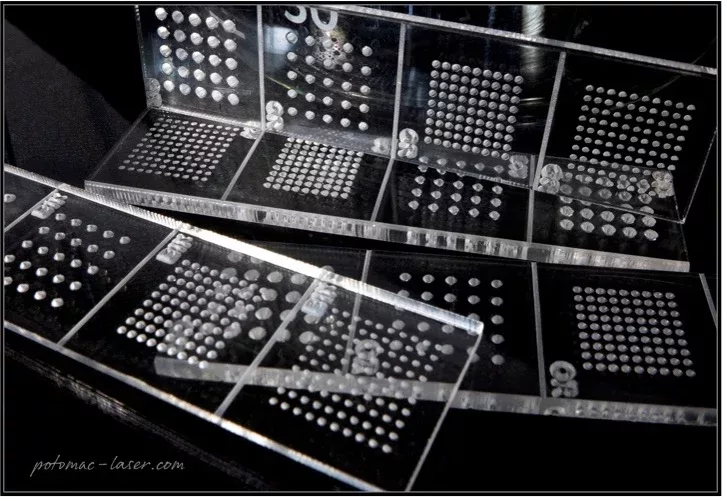
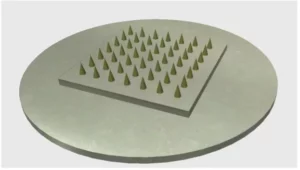
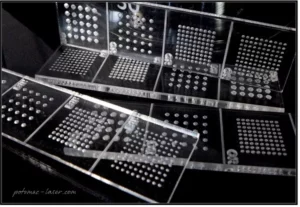
The images shown here are microneedle molds (diameters of 250 µm to 1 mm) prepared using Potomac’s precision laser etching technologies.
References:
- Tuan-Mahmood, T.-M. et al. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. 50, 623–37 (2013).
- Rouphael, N. G. et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 390, 649–658 (2017).