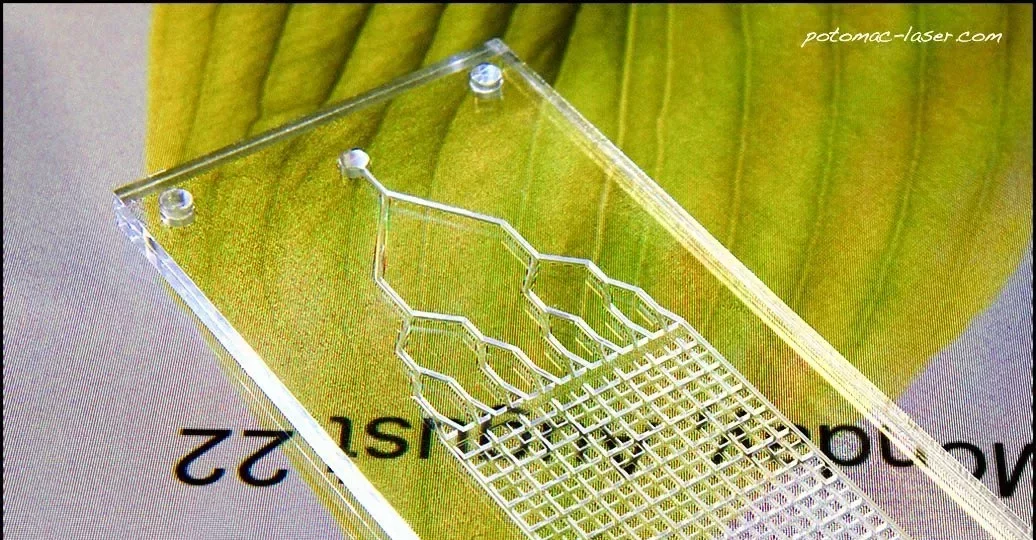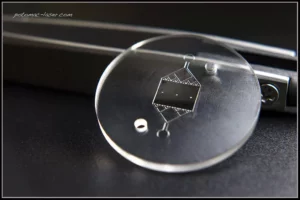
March 2020, Astek Diagnostics LLC, won the Maryland Innovation Initiative (MII) Award to develop a microfluidic device to detect bacterial infections in blood and evaluate the antimicrobial susceptibility. Astek, in partnership with Potomac Photonics Inc., will be designing a state-of-the art microfluidic lab-on-chip sensor for rapid detection of microorganisms in bodily fluids (like, blood, urine, tear drops and saliva). The microfluidic device design, rapid bacterial testing, and translation into the clinic will be conducted at the University of Maryland, Baltimore, Medical School.
The antimicrobial resistance (AMR) crisis which is predicted to become the number one cause of death by 2050, resulting to nearly 10 million deaths per year unless we mount a global response1. The countermeasures for AMR are more effective sensing and early detection of mutations within microorganisms. Current detection of antimicrobial susceptibility testing measurements relies largely on cell culture-based assays that are costly, labor intensive and can take anywhere from 2-3 days. Astek Diagnostics, has developed a cost-effective technology capable of analyzing contaminant within 60 minutes2. Such technology has various implication in healthcare, homeland security and the pharmaceutical industry, like for example, combating sepsis, environmental control for homeland security, infectious diseases, contaminants in food and beverages, biotherapeutic quality control, oil and gas industry, etc.
The microfluidic device prototyping, scale-up, manufacturing and commercialization of the product in the USA and other world markets will be dealt with by Potomac Photonics Inc. Our partnership will revolutionize the way we sense bacteria, disrupts the $18 billion cell culture industry, provides a cost-effective solution for 1.2 billion tests performed annually and most of all reduces the sensing time from days to minutes. Together with the Research and Development labs at Potomac Photonics Inc., researchers at Astek, look to commercialize this technology for Sepsis and AMR applications.
Potomac has significant experience in design and mass-production of microfluidic devices for biomedical application. Potomac’s Product and Process Technology (PPT) will lead this effort to help commercialize this technology for microbial bioburden and AMR detection at the Point-of-care. Potomac Photonics Inc. specializes in various microfluidics device fabrication methods, with client services for feature sizes ranging from 2 mm – 2 mm and provide a wide range of capabilities with high precision multiplexing options. Together with the UMBC and the FDA, Potomac Photonics will cost-effective manufacturing solutions using thermoplastic devices and provide a path towards seamless manufacturing
References:
- Leonard, H. et al. Rapid diagnostic susceptibility testing of bacteria and fungi from clinical samples using silicon gratings. 1089504, 3 (2019).
- Al-Adhami, M., Tilahun, D., Rao, G., Gurramkonda, C. & Kostov, Y. Rapid Detection of Microbial Contamination Using a Microfluidic Device BT- Biosensors and Biodetection: Methods and Protocols Volume 1: Optical-Based Detectors. in (eds. Rasooly, A. & Prickril, B.) 287–299 (Springer New York, 2017). doi:10.1007/978-1-4939-6848-0_18.