Joe La Fiandra
University of Maryland College Park (UMCP)
Major: Bioengineering
Minor: Global Engineering Leadership
Microfluidic Concentration Gradient Generators
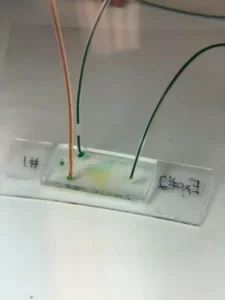 Many biochemical processes in biological organisms are controlled by concentration gradients. From cell migration to drug proliferation, concentration gradients are integral to the transportation of a vast array of substances throughout the body. Until recently, concentration gradient generation (CGG) platforms were simply too large to control molecular diffusion of reagents, resulting in premature comingling of fluids (source). Because of their prohibitive size, previous CGGs prevented identical replication of volumes and flow rates found in vivo, thereby tainting experimental data relevant to microscopic biochemical gradients. Therefore, modeling gradients on a microscopic scale is extremely valuable to understanding these complex interactions and the mechanics that enable them.
Many biochemical processes in biological organisms are controlled by concentration gradients. From cell migration to drug proliferation, concentration gradients are integral to the transportation of a vast array of substances throughout the body. Until recently, concentration gradient generation (CGG) platforms were simply too large to control molecular diffusion of reagents, resulting in premature comingling of fluids (source). Because of their prohibitive size, previous CGGs prevented identical replication of volumes and flow rates found in vivo, thereby tainting experimental data relevant to microscopic biochemical gradients. Therefore, modeling gradients on a microscopic scale is extremely valuable to understanding these complex interactions and the mechanics that enable them.
With innovative rapid-prototyping technologies, Potomac Photonics has developed a process for manufacturing microfluidic concentration gradient generators (µ-CGGs). Constructed on a polydimethylsiloxane (PDMS) substrate bonded to glass, this device provides a low cost, easily executable method of investigating gradient-driven mechanisms. This platform leverages laminar flow, a fluid dynamic phenomena, and fluid dynamic geometries required to control the movement of reagents. In the µ-CGG, serpentine features allow for controlled diffusion of the reagents, forming mixtures of varying concentrations throughout the device. Designed with a tree-shaped network, the µ-CGG replicates the experiment 39 times at varying concentrations all on the same chip to create the gradient seen in the image. Using flow rates and volumes on a micro order of magnitude, µ-CGGs mimic a nearly-identical environment to that of a biochemical concentration gradient naturally found in biological organisms.
While this µ-CGG design utilizes only 39 nodes in its flow network, the versatility, design potential, and low-cost of microfluidic device fabrication allow for a plethora of adjustments to complicate or simplify a µ-CGG. The sheer size of a microfluidic device is enough to enable multi-layer µ-CGGs to contain multiple gradients that flow into each other, or different flow levels resulting in dozens of combinations of several reagents. The ease of manufacturing µ-CGGs is particularly valuable. Primarily made out of elastomers and thermoplastics, µ-CGGs can be manufactured in substrates with specific material properties that enable complex chemical reactions to occur for relatively low cost. Leveraging all of these attributes, engineers can design µ-CGGs to their unique specifications on a single chip, decreasing time, materials, and personnel necessary for experimental set up and increasing volume of accurate data on a concise device.